As part of our strategic plan, Investing in Discovery, we pledged to “expand and extend the NIGMS commitment to facilitating the development of a diverse and inclusive biomedical research workforce” and “adopt a comprehensive, systems-based approach to address future workforce development issues.” In keeping with these goals, we convened a workshop to examine the benefits and feasibility of developing computational models of the biomedical workforce that would aid in program development and evaluation. Based on the discussions, we issued a new request for applications last week to develop computational models of U.S. scientific workforce dynamics. I encourage individuals or groups who are interested in this challenging area to consider applying, and I and others at NIGMS are looking forward to interacting with these researchers once awards are made.
The scientific workforce was also a focus of last week’s address by President Obama to the National Academy of Sciences. He made many important points about the ways that science impacts society, as well. I have included several excerpts below.
The President spoke of the potential impact of basic research, the need to support it and its benefits, saying:
No one can predict what new applications will be born of basic research: new treatments in our hospitals, or new sources of efficient energy; new building materials; new kinds of crops more resistant to heat and to drought.
History also teaches us the greatest advances in medicine have come from scientific breakthroughs, whether the discovery of antibiotics, or improved public health practices, vaccines for smallpox and polio and many other infectious diseases, antiretroviral drugs that can return AIDS patients to productive lives, pills that can control certain types of blood cancers, so many others.
Because of recent progress — not just in biology, genetics and medicine, but also in physics, chemistry, computer science, and engineering — we have the potential to make enormous progress against diseases in the coming decades.
As you know, scientific discovery takes far more than the occasional flash of brilliance — as important as that can be. Usually, it takes time and hard work and patience; it takes training; it requires the support of a nation. But it holds a promise like no other area of human endeavor.
The President challenged scientists to “use your love and knowledge of science to spark the same sense of wonder and excitement in a new generation,” adding:
So I want to persuade you to spend time in the classroom, talking and showing young people what it is that your work can mean, and what it means to you. I want to encourage you to participate in programs to allow students to get a degree in science fields and a teaching certificate at the same time. I want us all to think about new and creative ways to engage young people in science and engineering, whether it’s science festivals, robotics competitions, fairs that encourage young people to create and build and invent — to be makers of things, not just consumers of things.
He also spoke of the need to “create research opportunities for undergraduates and educational opportunities for women and minorities who too often have been underrepresented in scientific and technological fields, but are no less capable of inventing the solutions that will help us grow our economy and save our planet.”
I recommend taking the time to watch, listen to or read his entire presentation.




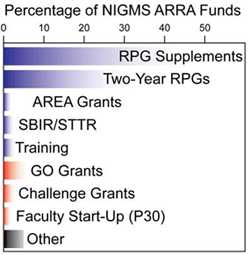 Applications in the first five categories (blue) were submitted and reviewed prior to the Recovery Act. The next three categories (red) are Recovery Act programs that solicited new applications, which are now being reviewed. The final category (black) includes supplements to research centers, research management and support and some other programs.
Applications in the first five categories (blue) were submitted and reviewed prior to the Recovery Act. The next three categories (red) are Recovery Act programs that solicited new applications, which are now being reviewed. The final category (black) includes supplements to research centers, research management and support and some other programs.
 NIH’s draft guidelines for human stem cell research (link no longer available) are now published in the Federal Register for
NIH’s draft guidelines for human stem cell research (link no longer available) are now published in the Federal Register for