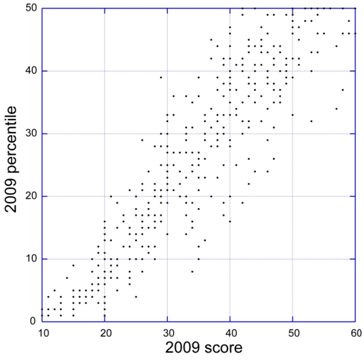
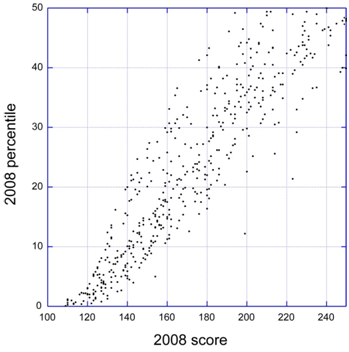
NIH announced this week that the 2-day “error correction window” to fix NIH system-identified errors or warnings after the submission deadline is being eliminated (see NOT-OD-10-123). This change will take effect for submission deadlines on or after January 25, 2011. You will still have up to 2 business days to view the application image and submit a corrected/changed application, as long as you do so before the deadline.
The error correction window was instituted by NIH as a temporary measure to facilitate the transition from paper to electronic applications.
In light of this change and another related to post-submission materials, it is really important to make sure that you submit an application early (before the submission deadline) so that you and your signing official have an opportunity to address any errors or warnings.
NIH’s Applying Electronically Web site includes many helpful resources, such as tips for avoiding common errors.



 NIH has announced a significant upgrade to the citation management capability of investigators’ personal profiles in the eRA Commons.
NIH has announced a significant upgrade to the citation management capability of investigators’ personal profiles in the eRA Commons.