One of my jobs as an NIGMS program director is helping investigators navigate the review process. This includes understanding where their applications will be reviewed and how they can make a recommendation about this assignment.
Applications can be grouped for review by research area or grant mechanism, or as a cohort submitted in response to a specific funding opportunity announcement (FOA). I would like to briefly walk through a few scenarios and share some advice along the way.
The “Review and Selection Process” (V.2) section of FOAs provides clues about where your application will be reviewed. An application can be reviewed at the Center for Scientific Review (CSR) or at an individual NIH institute or center (IC), depending upon the particular FOA. Information about who will review your application is posted in your eRA Commons account soon after it is determined, but you should contact your program director or CSR if you have questions or concerns.
The vast majority of applications received by NIH on topics relevant to NIGMS are in response to “parent” program announcements, such as PA-13-302, for unsolicited R01 applications. Section V.2 of PA-13-302 states, “Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by CSR.” This means that the application will be reviewed by a regular NIH study section or a special emphasis panel (SEP) with expertise in the research area explored by the application. To identify a possible “review home” for your application, I suggest you peruse CSR’s list of study sections, find the ones that seem most suitable for your application and then use NIH RePORTER to search for funded applications that have been reviewed by those study sections. This will allow you to identify the group of scientists who have the appropriate research expertise to review your application.
Specific requests for applications (RFAs), such as RFA-GM-14-003 (Revisions for Macromolecular Interactions in Cells), are often reviewed together in the IC that issued the RFA. For example, section V.2 of GM-14-003 states, “Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by the NIGMS.” This means that the NIGMS Office of Scientific Review will organize a review panel to review applications submitted in response to this RFA.
For applications that will be reviewed by groups convened by CSR, I encourage the investigators I speak with to write a brief cover letter for their applications that indicates which study section they think is appropriate and how they arrived at that conclusion. Sometimes, it is also helpful to indicate the type(s) of expertise you believe is needed to review your application, but you should not provide a list of reviewers, as that creates issues with potential conflicts of interest. The recommendations made in the cover letter are advisory, but the CSR Division of Receipt and Referral makes every effort to accommodate reasonable requests.


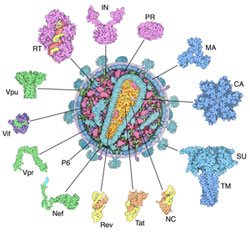

 kicked off December 11 at the Philadelphia Convention Center. The keynote symposium began with Gary Borisy’s description of the first ASCB meeting, when Hans Ris described his then-heretical finding that chloroplasts contain DNA. For more details about the genesis and early years of the ASCB, check out John Fleishman’s article, A Place of Our Own, in the December 2010 ASCB Newsletter.
kicked off December 11 at the Philadelphia Convention Center. The keynote symposium began with Gary Borisy’s description of the first ASCB meeting, when Hans Ris described his then-heretical finding that chloroplasts contain DNA. For more details about the genesis and early years of the ASCB, check out John Fleishman’s article, A Place of Our Own, in the December 2010 ASCB Newsletter.