The August issue of NIH’s Extramural Nexus includes two announcements that might interest you.
The August issue of NIH’s Extramural Nexus includes two announcements that might interest you.
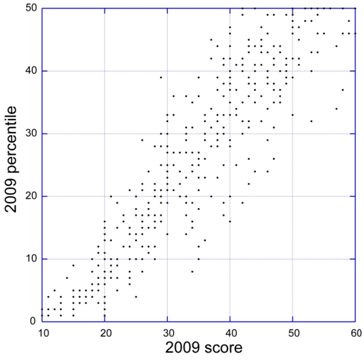
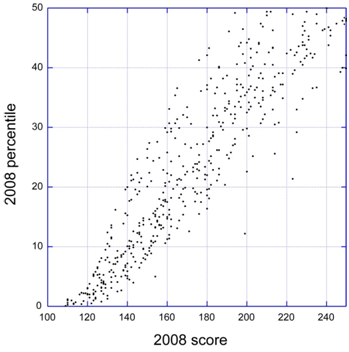
Impact Score Paragraph in Summary Statements
Starting with September grant application reviews, reviewers will include a summary paragraph to explain what factors they considered in assigning the overall impact score. This should help investigators better understand the reasons for the score.
Plain Language in Public Sections of Grant Applications
The director’s column talks about the importance of communicating research value in your grant application.
Your grant title, abstract and statement of public health relevance are very important. Once a grant is funded, these items are available to the public through NIH’s RePORTER database. Many people are interested in learning about research supported with taxpayer dollars, so I encourage you to be clear and accurate in writing these parts of your application. Reviewers are being told to expect plain language in these sections.
The Nexus column includes links to these helpful resources:


 NIH has announced a significant upgrade to the citation management capability of investigators’ personal profiles in the eRA Commons.
NIH has announced a significant upgrade to the citation management capability of investigators’ personal profiles in the eRA Commons.