You may be interested in the following recently issued funding opportunity announcements:
Collaborations for Macromolecular Interactions in Cells (R01)
(RFA-GM-13-004)
Purpose: Establish interdisciplinary collaborative projects to advance studies of macromolecular interactions and their relationship to function in cells.
Letter of intent due date: May 14, 2012
Application due date: June 14, 2012
NIGMS contacts:
Division of Biomedical Technology, Bioinformatics, and Computational Biology
Paul Brazhnik, 301-451-6446
Division of Cell Biology and Biophysics
James Deatherage and Alexandra Ainsztein, 301-594-0828
Division of Genetics and Developmental Biology
Daniel Janes, 301-594-0943
Division of Pharmacology, Physiology, and Biological Chemistry
Vernon Anderson, 301-594-3827
Research Networks for Macromolecular Interactions in Cells (U54)
(RFA-GM-13-005)
Purpose: Establish interdisciplinary collaborative research networks to advance studies of macromolecular interactions and their relationship to function in cells.
Letter of intent due date: May 14, 2012
Application due date: June 14, 2012
NIGMS contacts:
Division of Biomedical Technology, Bioinformatics, and Computational Biology
Paul Brazhnik, 301-451-6446
Division of Cell Biology and Biophysics
James Deatherage and Alexandra Ainsztein, 301-594-0828
Division of Genetics and Developmental Biology
Daniel Janes, 301-594-0943
Division of Pharmacology, Physiology, and Biological Chemistry
Vernon Anderson, 301-594-3827
Small Business Alzheimer’s Disease Research (SBIR[R43/R44])
(RFA-OD-12-003)
Purpose: Solicit Small Business Innovation Research (SBIR) grant applications in the area of Alzheimer’s disease.
Letter of intent due date: March 30, 2012
Application due date: April 30, 2012
NIGMS contact: Scott Somers, 301-594-3827
Small Business Alzheimer’s Disease Research (STTR[R41/R42])
(RFA-OD-12-004)
Purpose: Solicit Small Business Technology Transfer Research (STTR) grant applications in the area of Alzheimer’s disease.
Letter of intent due date: March 30, 2012
Application due date: April 30, 2012
NIGMS contact: Scott Somers, 301-594-3827


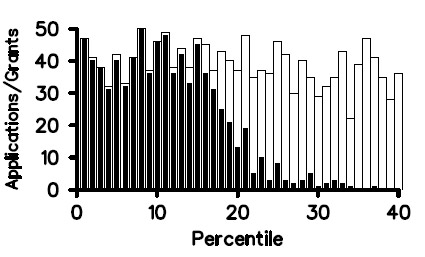
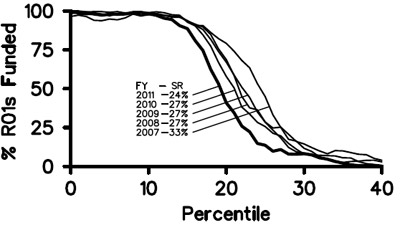
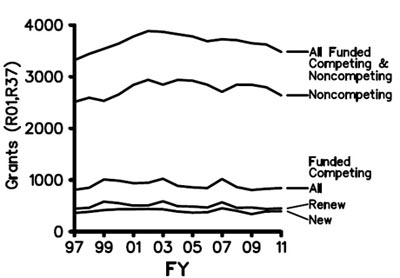
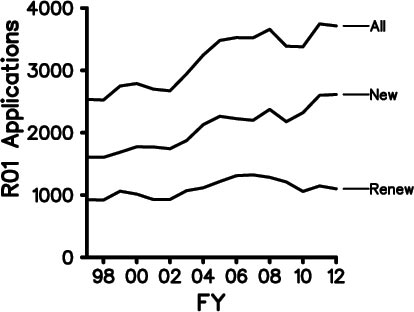
 It’s been nearly a year since we posted our Strategic Plan for Biomedical and Behavioral Research Training. In August, I announced that we were on course to implement most of the plan in early 2012. I’m very pleased to tell you that our Blueprint for Implementation is now available. As you’ll see, it’s truly a blueprint, and in the months ahead we’ll be posting more details and guidance about each of the action items.
It’s been nearly a year since we posted our Strategic Plan for Biomedical and Behavioral Research Training. In August, I announced that we were on course to implement most of the plan in early 2012. I’m very pleased to tell you that our Blueprint for Implementation is now available. As you’ll see, it’s truly a blueprint, and in the months ahead we’ll be posting more details and guidance about each of the action items.