I would like to call your attention to two program announcements recently published in the NIH Guide:
- PAR-17-315: Pre-application for a Biomedical Technology Research Resource (X02)
Due: August 15, 2017
- PAR-17-316: Biomedical Technology Research Resource (P41)
Due: September 25, 2017
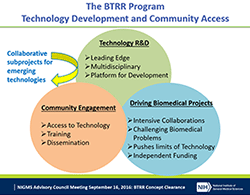
These announcements provide updated instructions for both pre-applications and full applications for Biomedical Technology Research Resource (BTRR) grants. The BTRR program supports development and dissemination of advanced technologies that enable biomedical research The BTRR centers create a wide range of technologies and work with thousands of NIH-supported investigators each year.
The X02 pre-application is strongly recommended. The pre-application provides an opportunity for prospective applicants to receive feedback from both peer reviewers and NIGMS program staff as they formulate their plans for a complex, lengthy proposal for a P41 grant.
Following an evaluation in 2016, we have revised the BTRR program, while preserving the fundamental mission of developing and providing access to advanced technologies. Susan Gregurick, director of our Biomedical Technology, Bioinformatics, and Computational Biology Division, presented on the evaluation and proposed program changes at the September 2016 NIGMS Advisory Council meeting.
Revisions to the program have changed the structure of a BTRR to give the investigators who run the centers more flexibility in how technologies are shared with the community. A new feature, “Technology Development Partnerships,” will enable centers to rapidly adopt and incorporate emerging technologies developed elsewhere that advance a BTRR’s overall mission, rather than focus entirely on technologies developed “in-house.”
The program also will provide investigators with greater flexibility to tailor a center’s approach to technology innovation, user access and training, and dissemination according to the specific technologies being developed and communities being served. At the same time, the program will place a greater emphasis on actively moving technologies out of the BTRR and into the wider community as quickly as possible. We anticipate that most BTRR centers will not be funded beyond three cycles (15 years), and we will require investigators involved with this program to formulate a sustainability plan for their research resources.
The submission date for the first round of X02 pre-applications is August 15, 2017. Future submission dates will follow a regular schedule, occurring twice per year in March and July. That timing allows nine months from submission of the X02 until the anticipated submission of a resulting full application in January or May, respectively.
The next submission date for full applications for a P41 BTRR is September 25, 2017. This is the only submission date for funding in Fiscal Year 2018. In future years, applications will be accepted twice per year, in January and May, with no September submission. To improve consistency in the review of competing applications, the NIH Center for Scientific Review will convene a special study section to review all NIGMS P41 BTRR applications together. There will be no site visits.
NIGMS also supports technology development through several other programs. To help investigators determine which technology development program is right for their project, we’ve posted a decision tree on the NIGMS website. It includes descriptions of the programs designed to support specific stages of technology development.
I welcome questions or comments about these FOAs or our technology development programs in general.