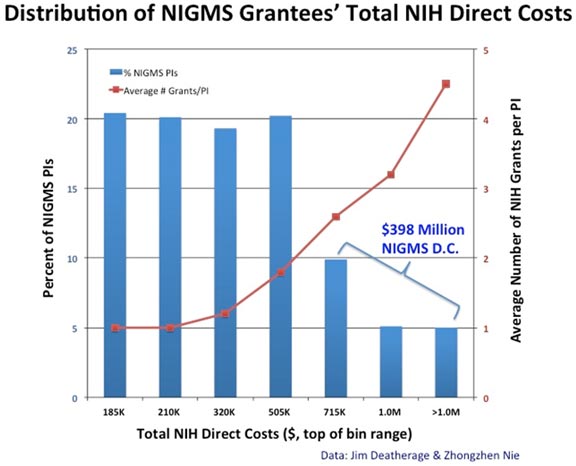
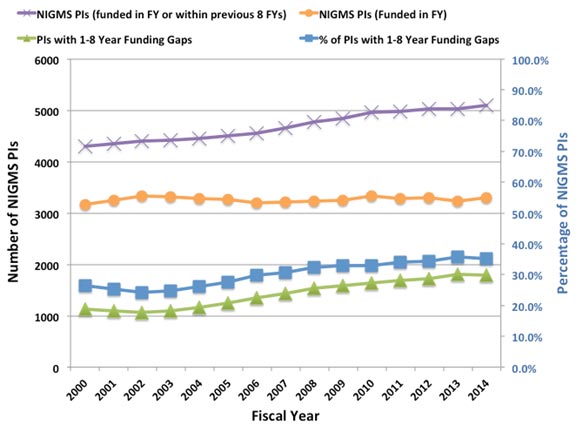
We’re planning an experiment in how we fund research, and we want your input. As outlined in the Request for Information (RFI) included below, we propose to create a pilot program called Maximizing Investigators’ Research Award (MIRA) that would support all of the projects in an investigator’s lab that are relevant to the NIGMS mission.
We expect that the MIRA program will offer a number of benefits. For instance, investigators would not have to break their work into smaller, strictly prescribed increments. In addition, the program could improve funding stability and enhance grantees’ flexibility to follow new research directions as opportunities and ideas arise.
It’s important to note that MIRAs are not intended to be a method for supporting only a perceived elite group of investigators or promoting only high-risk, high-potential-reward research.
Our intent is to pilot a program that might transform how we support fundamental biomedical research, creating a more productive, efficient and sustainable enterprise. I encourage you to read the proposal and share your comments using the RFI input form (no longer available) by the August 15 deadline. We welcome responses from both individuals and organizations.
Request for Information (RFI): Soliciting Comments on a Potential New Program for Research Funding by the National Institute of General Medical Sciences
Notice Number: NOT-GM-14-122
Key Dates
Release Date: July 17, 2014
Response Date: August 15, 2014
Related Announcements
None
Issued by
National Institute of General Medical Sciences (NIGMS)
Purpose
This is a time-sensitive Request for Information (RFI) directed at obtaining input to assist the National Institute of General Medical Sciences (NIGMS) in its planning for a potential new program tentatively named Maximizing Investigators’ Research Award (MIRA). This award would be a grant in support of all of the research supported by NIGMS in an investigator’s laboratory.
Background
Supporting basic research by funding individual projects has a number of consequences for the efficiency and effectiveness of the basic biomedical research enterprise in the U.S. (Alberts, 1985; Ioannidis, 2011; Vale, 2012; Bourne, 2013; Alberts et al., 2014). To address these issues and increase the efficiency and efficacy of its funding mechanisms, NIGMS is considering a pilot program to fund investigators’ overall research programs, which represents a compilation of the investigator’s research projects. It is hoped that this new funding mechanism will achieve the following:
- Increase the stability of funding for NIGMS-supported investigators, which could enhance their ability to take on ambitious scientific projects and approach problems creatively.
- Increase flexibility for investigators to follow important new research directions as opportunities arise, rather than being bound to specific aims proposed in advance of the studies.
- Improve the distribution of funding among the nation’s highly talented and promising investigators to increase overall scientific productivity and the chances for important breakthroughs.
- In the long term, reduce the time spent by researchers writing and reviewing grant applications, allowing them to spend more time conducting research.
Overview of the proposed NIGMS MIRA program
- An NIGMS MIRA would provide support for a lab’s research program, which represents a compilation of the investigator’s NIGMS research projects (research areas supported by NIGMS are outlined at our website). Researchers would have the freedom to explore new avenues of inquiry that arise during the course of their work as long as those avenues are relevant to the mission of the Institute and do not require additional review for regulatory compliance (e.g., new human subjects research).
- An NIGMS MIRA would be renewable.
- Funding would range from $150,000-$750,000 (direct costs/year), depending on recommendations of the study section and the National Advisory General Medical Sciences Council as well as staff evaluation of the needs and expected productivity and impact of the program. Support for the investigator from sources other than NIGMS would be taken into consideration when deciding on funding levels for an NIGMS MIRA.
- Up to $150,000 in administrative supplement support for the purchase of new equipment could be requested by an NIGMS MIRA grantee per grant cycle. Decisions on these requests would be made by NIGMS staff and the National Advisory General Medical Sciences Council based on an assessment of need and the potential impact of the new equipment on the research. The number of supplements given would depend on the available funds.
- The median direct costs for NIGMS MIRAs would be higher than the current median R01 direct costs at NIGMS.
- The length of an NIGMS MIRA would be 5 years, which is longer than the current average for an NIGMS R01 of close to 4 years.
- A researcher funded by an NIGMS MIRA would not be given any other sources of NIGMS funding with the following exceptions:
- Grants supporting research resources
- Grants supporting training, workforce development or diversity building
- Funding for clinical trials
- SBIR/STTR grants
- Conference grant
- The Program Director/Principal Investigator (PD/PI) would be expected to commit at least 50 percent research effort to the NIGMS MIRA.
- Revision applications to allow new collaborative work might be included as part of this program.
- Review of the application would emphasize a holistic evaluation of the investigator’s track record and the overall potential importance of the proposed research program, without focusing on specific project details. Specific aims would not be required. The process would include peer review using existing criteria and processes but would be tailored to address the particular features of the MIRA (see the section below on the Possible Peer Review Process).
- To avoid the abrupt termination of research groups from an adverse round of peer review, NIGMS MIRAs could be ramped down from one funding level to a lower one that is more consistent with the recent and perceived future productivity of the group and the importance of the work, as assessed by the study section. Conversely, a renewing program could have its budget increased if the perceived productivity, impact and needs merited it. As per standard grants policy, NIGMS program staff would make decisions on funding levels, guided by the recommendations from study sections and the National General Medical Sciences Council.
Possible Peer Review Process
In addition to the standard review criteria, among the considerations reviewers would be asked to address in reviewing MIRA applications are whether:
- The proposed research effort is substantive, broad and ambitious.
- A PD/PI’s record shows evidence of productivity, creativity, adaptability, service and excellence in mentoring.
- For Early stage Investigators (ESIs), there is evidence of productivity, independent research and contributions to the design and direction of past research efforts.
- The proposed research includes evidence of creativity and the incorporation of novel approaches as appropriate.
- There are sound bases and generally well-thought-through and reasoned approaches for the proposed research effort.
- There is evidence that the PD/PI has considered alternative approaches, outcomes, models and directions that might inform the scientific questions being posed.
- The work will be conducted carefully and cost-effectively, with good stewardship of the data generated.
- For ESIs, there is evidence of institutional support and mentoring.
Possible Implementation Plan
Because this is a pilot program, implementation must be carefully phased in and outcomes and unintended consequences assessed along the way. One possible implementation plan, consisting initially of two paths, is outlined below.
- In lieu of a competitive renewal (Type 2), PDs/PIs who currently have two or more NIGMS R01s could apply for an NIGMS MIRA. Application for a MIRA would be evidence of a willingness to relinquish all other NIGMS research grants upon award. Award of the MIRA would be contingent on relinquishing other current NIGMS research grants in favor of the MIRA. Applicants proposing to consolidate their NIGMS awards would have to submit a MIRA application that would undergo peer review. The budget would be higher than that for any of the individual awards the PD/PI has, but usually less than the total of all of his or her NIGMS support. The length of the NIGMS MIRA would be 5 years.
- The program could be open to applications from ESIs. This would bring a cadre of ESIs into the system who could be directly compared to other NIH-supported ESIs funded through traditional mechanisms. MIRA would be considered a substantial, independent NIH research award that disqualifies an individual from classification as an ESI.
The MIRA Funding Opportunity Announcement, when ultimately published, will include metrics that will be used to evaluate the success of the program. Once the program is established and indications of success have been measured, additional groups of investigators would be invited to apply for NIGMS MIRAs. If the program becomes successful, it would ultimately be open to applications from all investigators working on topics relevant to the mission of NIGMS and could become the primary research funding mechanism used by the Institute.
Information Requested
NIGMS is planning to issue a Funding Opportunity Announcement (FOA) to test this new program on a pilot scale. To aid in planning, the Institute is seeking feedback from the scientific community. NIGMS invites comments on the topics below; however, comments are not limited to these topics.
- The merits of this funding program for established and early stage investigators.
- The likelihood that established and early stage investigators would apply for NIGMS MIRAs.
- Concerns about the NIGMS MIRA proposal.
- Suggestions for changes to improve the NIGMS MIRA proposal or associated processes.
Submitting a Response
All responses must be submitted to https://www.research.net/s/NewGrantProgram_gov (no longer available) by August 15, 2014. Responses are limited to 500 words per topic.
This RFI is for planning purposes only and should not be construed as a solicitation for applications or an obligation on the part of the government. The government will not pay for the preparation of any information submitted or for the government’s use of that information.
The NIH will use the information submitted in response to this RFI at its discretion and will not provide comments to any responder’s submission. However, responses to the RFI may be reflected in future funding opportunity announcements. The information provided will be analyzed and may appear in reports. Respondents are advised that the Government is under no obligation to acknowledge receipt of the information or provide feedback to respondents with respect to any information submitted. No proprietary, classified, confidential, or sensitive information should be included in your response. The Government reserves the right to use any non-proprietary technical information in any resultant solicitation(s).
Inquiries
Please direct all inquiries to:
Peter C. Preusch, Ph.D.
National Institute of General Medical Sciences (NIGMS)
Telephone: 301-594-0827
Helen R. Sunshine, Ph.D.
National Institutes of General Medical Sciences (NIGMS)
Telephone: 301-594-2881


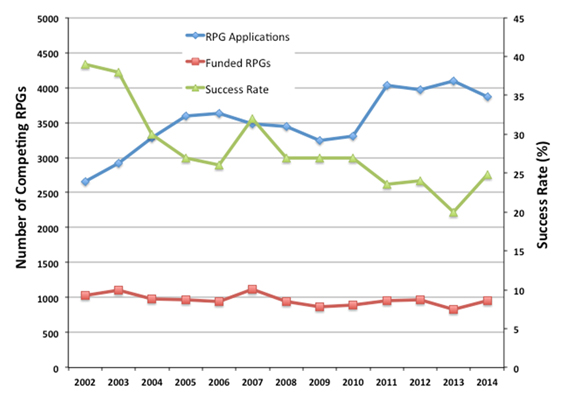
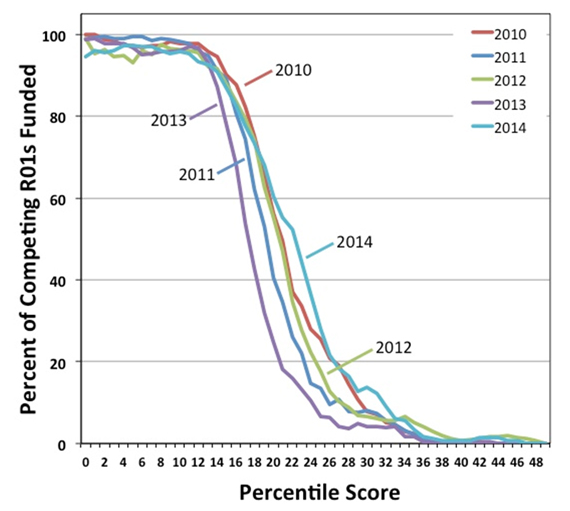
 I’m delighted to tell you that Judith Greenberg is NIGMS’ new deputy director.
I’m delighted to tell you that Judith Greenberg is NIGMS’ new deputy director.