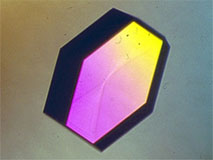
A crystal of hen egg white lysozyme. Credit: Alex McPherson, University of California, Irvine.
As you may know from the coverage in various popular news outlets and science journals (see below for a list), the United Nations Educational, Scientific and Cultural Organization has declared 2014 to be the “International Year of Crystallography.” This is in recognition of the 100th anniversary of the Nobel Prize in physics to Max von Laue for the discovery of diffraction of X-rays by crystals.
I first learned about crystallography in college, when I took a course in physical chemistry that included an introduction to chemical crystallography. Crystallography combines math, computer science, chemistry and biology—and that’s what convinced me to do graduate work in the field. When you determine structures, you’re often the first person to ever see that molecule, and that’s pretty exciting.
Since 1914, scientists have made many advances in the use of X-rays for the atomic-level determination of the 3-D structure of molecules. For instance, in the early years of the 20th century, William and Lawrence Bragg, father and son, learned that the newly discovered X-ray radiation could be used to locate the atoms in a crystal of matter. Their work ultimately led to Bragg’s Law for understanding X-ray diffraction and the structure determination of materials ranging from table salt to the ribosome. Breakthroughs made possible by crystallography (and diffraction) have led to 15 Nobel Prizes, including 7 with NIGMS support.
X-ray crystallography has impacted all areas of science, including biomedical research. The first biological finding was made by James Sumner, who discovered that enzymes could be crystallized (urease was the enzyme). In the years following, X-ray diffraction and crystallography have been used to reveal the structure of DNA and countless proteins and enabled structure-based drug design efforts. Crystallography has become an established tool of small molecule and protein studies.
Today, modern biological crystallography is practiced at synchrotron facilities, with access to 20 X-ray crystallography beamlines supported entirely or in part by NIGMS. Efforts are under way to make smaller, more intense beamlines that will allow the study of small crystals. Crystallography is also very much a part of the new X-ray laser facilities, where several NIGMS investigators are carrying out pioneering research on very small crystals of proteins, including membrane proteins, on X-ray scattering of proteins in solution and on protein dynamics.
Clearly, crystallography continues to be a cutting-edge field, and I’m excited to see what advances it brings during the coming years.
Articles about the International Year of Crystallography:
January 30 Special Issue of Nature
UN to Raise Awareness of Little-Known Science Behind DNA, Computer Memory, New Drugs
2014 Is the International Year of Crystallography (‘What’s Crystallography?’ You Ask)



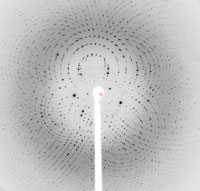
 Over the course of its 10-year existence, the Protein Structure Initiative has supported the development of new technologies and methods that improve the throughput of protein structure determination. Many of them apply to the production of purified proteins for functional and structural studies, and you don’t need access to major research centers to use them.
Over the course of its 10-year existence, the Protein Structure Initiative has supported the development of new technologies and methods that improve the throughput of protein structure determination. Many of them apply to the production of purified proteins for functional and structural studies, and you don’t need access to major research centers to use them.