The successful entry and retention of new investigators into biomedical research is a priority for us, and the renewal rate of this group’s first R01 research grants is an important indicator for this goal. Here are the results of an analysis I did of the first competing renewal rates for new and established investigators.
Figure 1 shows that the first competing renewal rate of new investigators’ first NIGMS R01 or R29 grants has declined over the past 10 years. This trend is similar to the one for overall NIGMS R01 application success rates.
Figure 1. Percentage of new investigators’ R01 and R29 grants that were successfully renewed. The horizontal axis is the fiscal year in which the first project period ended. The vertical axis is the percentage of these projects that were successfully renewed at least once (regardless of whether the new or amended competing renewal application was funded) by the end of Fiscal Year 2014.
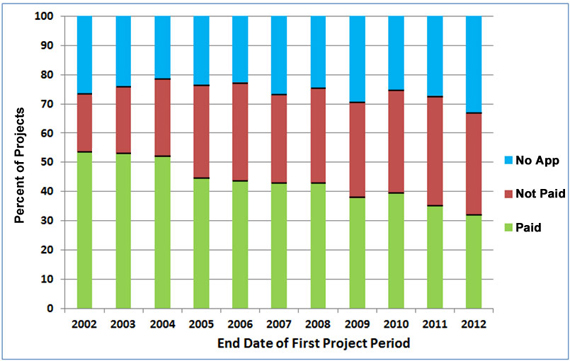
Figure 2 gives a more complete picture of the renewal history of new investigators’ NIGMS R01 and R29 projects. In addition to the renewal rate (also shown in Figure 1), it shows the percentage of projects for which at least one renewal application was submitted but was not successfully renewed as well as the percentage of projects for which no renewal application was submitted.
Figure 2. Renewal history of new investigators’ R01 and R29 grants between Fiscal Years 2002-2012. The bottom section (green) shows successful renewals (paid), which are also shown in Figure 1; the middle section (red) shows grants for which renewal was attempted but was not successful (not paid); and the top section (blue) shows grants for which no renewal application was submitted (no app).
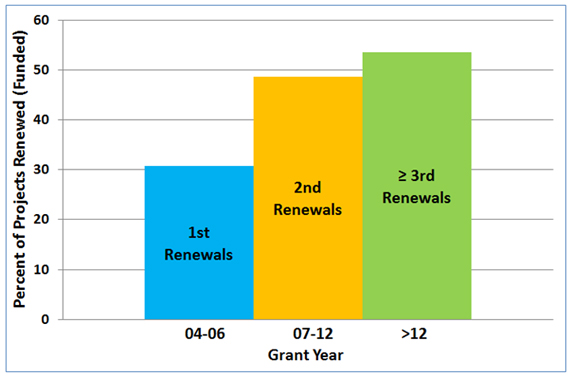
Figure 3 shows that success in renewing an NIGMS-funded R01 grant correlates positively with how long the grant has been active.
Figure 3. Competing renewal rates for the first, second and third or more renewals of NIGMS R01 grants awarded between Fiscal Years 2004-2007 to new and established investigators.
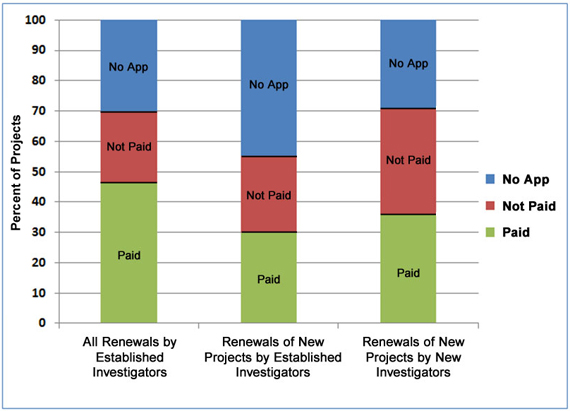
Since first renewals have lower success rates than subsequent renewals, Figure 4 addresses whether new investigators seeking to renew their first R01 grants are competitive with established investigators who are renewing long-term and/or new projects. The figure shows that the renewal rate for all projects from established investigators, including new as well as long-term projects, is higher than the renewal rate of projects from new investigators (46 percent in the left column versus 36 percent in the right column). However, when focusing only on the first renewals of new projects (in the middle and right columns), new investigators are renewing at a higher rate than are established investigators (36 percent versus 30 percent).
Figure 4. Renewal history of NIGMS R01 projects from new and established investigators that were initially funded by NIGMS between Fiscal Years 2004-2007.
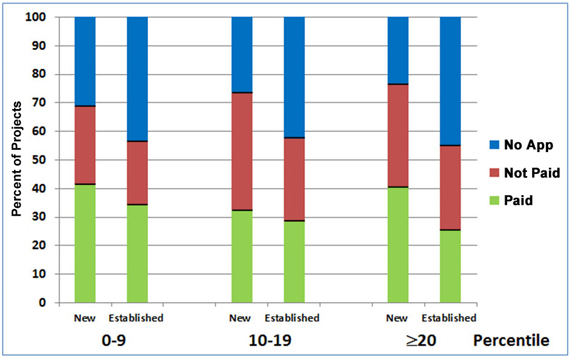
Figure 5 shows the relative success of new and established investigators in renewing new projects as a function of the percentile score obtained on the initial award. As the “Paid” sections of the bars indicate, for each of the percentile groups, the overall renewal rate for new investigators’ new R01s was higher than that for established investigators’ new R01s.
Figure 5. Renewal history of NIGMS R01 projects from new and established investigators that were initially funded between Fiscal Years 2004-2007 in relation to the percentile ranking (0-9th, 10-19th, and 20th and higher percentiles) of the original award. The number of projects in each of the six categories analyzed from left (new investigators, 0-9th percentile) to right (established investigators, ≥20th percentile) are: 239, 328, 299, 347, 172 and 102.
Recognizing the importance of new investigators in sustaining the vitality of biomedical research, we give special consideration to applications from them, and in some cases, we fund these applications at percentiles beyond those for most established investigators. The data in Figure 5 supports this practice by showing that the renewal rates of new investigators whose original applications scored at or above the 20th percentile are about the same as, or higher than, those for new and established investigators whose original applications scored in the 0-9th percentile range.
More About This Analysis
This analysis includes Recovery Act projects and excludes withdrawn applications and multi-principal investigator grants.
Definitions
R01 projects: Research project grants.
R29 projects: First Independent Research Support and Transition (FIRST) awards, R01-type research project grants awarded to new investigators available from 1987 to 1998.
Renewal rate: Percentage of grants that were successfully renewed by the date of this analysis (end of Fiscal Year 2014), regardless of whether a new or amended competing renewal application was funded.
Grant year: Grant year in which the renewal R01 application was submitted.
New investigator: An individual who has not previously competed successfully as a program director/principal investigator for a substantial NIH independent research award (see http://grants.nih.gov/grants/glossary.htm#NewInvestigator).
 As part of a series of NIH-wide initiatives to enhance rigor and reproducibility in research, we recently launched a Web page that will serve as a clearinghouse for NIH and NIH-funded training modules to enhance data reproducibility. Among other things, the site will house the products of grants we’ll be making over the next few months for training module development, piloting and dissemination.
As part of a series of NIH-wide initiatives to enhance rigor and reproducibility in research, we recently launched a Web page that will serve as a clearinghouse for NIH and NIH-funded training modules to enhance data reproducibility. Among other things, the site will house the products of grants we’ll be making over the next few months for training module development, piloting and dissemination.

 I recently had the opportunity to talk to Phil Bourne, NIH’s associate director for data science, about some of the current Big Data to Knowledge (BD2K) initiative activities. I asked him how they tie together his vision of a digital enterprise for biomedical research and how they might benefit NIGMS grantees.
I recently had the opportunity to talk to Phil Bourne, NIH’s associate director for data science, about some of the current Big Data to Knowledge (BD2K) initiative activities. I asked him how they tie together his vision of a digital enterprise for biomedical research and how they might benefit NIGMS grantees.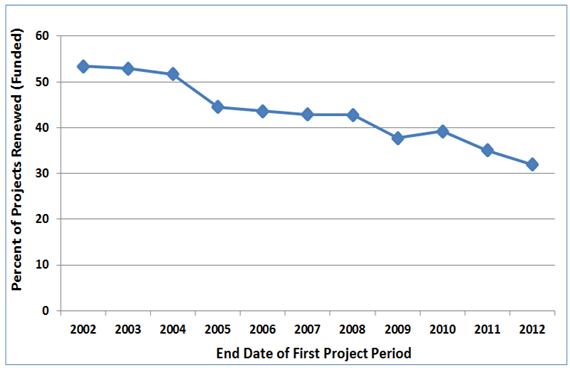
 NIGMS Director Jon Lorsch and I will field your questions about the recently announced Maximizing Investigators’ Research Award (MIRA) (R35) during a webinar on Thursday, Feb. 19, from 2-3 p.m. EST. You’ll be able to access the webinar at https://webmeeting.nih.gov/nigmsmira/. During the event, you can submit questions by calling
NIGMS Director Jon Lorsch and I will field your questions about the recently announced Maximizing Investigators’ Research Award (MIRA) (R35) during a webinar on Thursday, Feb. 19, from 2-3 p.m. EST. You’ll be able to access the webinar at https://webmeeting.nih.gov/nigmsmira/. During the event, you can submit questions by calling