Following up on the previous post regarding the first MIRA awards to New and Early Stage Investigators, we issued awards to a total of 94 grantees. In addition to ensuring that we are funding the highest quality science across areas associated with NIGMS’ mission, a major goal is to support a broad and diverse portfolio of research topics and investigators. One step in this effort is to make sure that existing skews in the system are not exacerbated during the MIRA selection process. To assess this, we compared the gender, race/ethnicity and age of those MIRA applicants who received an award with those of the applicants who did not receive an award, as well as with New and Early Stage Investigators who received competitive R01 awards in Fiscal Year (FY) 2015.
We did not observe any significant differences in the gender or race/ethnicity distributions of the MIRA grantees as compared to the MIRA applicants who did not receive an award. Both groups were roughly 25% female and included ≤10% of underrepresented racial/ethnic groups. These proportions were also not significantly different from those of the new and early stage R01 grantees. Thus although the MIRA selection process did not yet enhance these aspects of the diversity of the awardee pool relative to the other groups of grantees, it also did not exacerbate the existing skewed distribution.
We did observe significant differences among the mean ages of the MIRA grantees, MIRA applicants who did not receive an award and the R01-funded grantees. The MIRA grantees are 1.5 years younger on average than those MIRA applicants who did not receive an award (37.2 vs. 38.7 years, p<0.05), and about 2 years younger than the FY 2015 R01-funded Early Stage Investigators (37.2 vs. 39.1 years, p<0.001). The R01-funded New Investigators in FY 2015, a pool which includes a few individuals older than 60 years, average an age of 45.6 years. This selection for funding investigators earlier is a promising feature of the first round of MIRA awards to New and Early Stage Investigators. As noted at the recent meeting of our Advisory Council, where Jon presented these data, 37 years is still relatively late for investigators to be getting their first major NIH grant. We will continue to monitor this issue with the goal of further decreasing that figure.



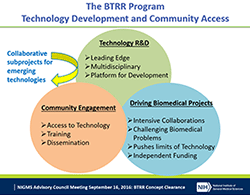


 At last week’s Advisory Council meeting, I presented a report on the comments we received in response to our request for information (RFI) on a potential new program for research funding.
At last week’s Advisory Council meeting, I presented a report on the comments we received in response to our request for information (RFI) on a potential new program for research funding.