At the September meeting of our National Advisory Council, I presented a concept clearance for our next step in response to the recommendations of the working group on sepsis. The videocast of the presentation is now available.
Continue reading “Human Biospecimen Collections for Sepsis Research”Tag: NIGMS Advisory Council
Advancing Sepsis Research: New Models and Novel Approaches
At the September meeting of the NIGMS Advisory Council, I delivered the Institute’s response to the recommendations of the Working Group on Sepsis. For more information, you can watch the videocast of the presentation.
Continue reading “Advancing Sepsis Research: New Models and Novel Approaches”Request for Information on Human Biospecimens for Research on Sepsis
NIGMS wants to advance our understanding of sepsis in order to accelerate improved diagnosis and treatment strategies. Based on the recommendations of our National Advisory General Medical Sciences Council Working Group on Sepsis, we intend to continue our support of fundamental discovery and mechanistic science relevant to sepsis. We recently identified the Institute’s specific priorities for sepsis research in an NIH Guide notice (NOT-GM-19-054). Additionally, the Working Group recommended that NIGMS encourage the use of human clinical materials to facilitate more rapid progress toward better identification, staging, and endotyping of the disease.
Continue reading “Request for Information on Human Biospecimens for Research on Sepsis”Notice of Information on NIGMS Priorities for Sepsis Research
At the recent National Advisory General Medical Sciences (NAGMS) Council meeting, the Recommendations of the NAGMS Working Group on Sepsis were presented. As one of our first responses to the recommendations of this working group, NIGMS has published a Notice of Information on NIGMS Priorities for Sepsis Research.
The Notice of Information provides potential applicants with details of specific research topics that are of special interest to NIGMS as well as those that are considered to be of low priority for funding.
If you have any questions about NIGMS Priorities for Sepsis Research, please contact me.
Recommendations of the NAGMS Working Group on Sepsis
Sepsis is a serious condition that affects about 1.7 million people and causes about 270,000 deaths annually in the U.S. Because it involves multiple organ systems, it is also one of the clinical research areas supported by NIGMS. Despite decades of research, sepsis remains a poorly understood condition with limited diagnostic tools or therapeutic interventions.
Nearly a year ago, we established a working group of our Advisory Council to advise us on how best to advance sepsis research. At last week’s Council meeting, Dr. John Younger and Dr. Monica Kraft, co-chairs of the working group, presented the group’s recommendations:
Continue reading “Recommendations of the NAGMS Working Group on Sepsis”Notice: Concept Clearance for MIDAS Coordination Center
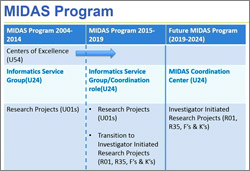
At its September 2017 meeting, our Advisory Council endorsed the concept of a MIDAS Coordination Center.
MIDAS, or the Models of Infectious Disease Agent Study program, is a collaborative network of research groups that focus on developing bioinformatics tools and computational models to understand the interactions between infectious agents and their hosts, disease spread, prediction systems, and response strategies.
Initially the MIDAS network consisted of research centers (U54s), research projects (U01s), and an information service group (U24). These activities will expire in 2019, and NIGMS is shifting the focus of this program to an investigator-initiated research portfolio consisting of R01s, R35 MIRA grants, and fellowships and mentored career development awards (Fs, Ks).
However, modeling of infectious disease agents continues to be an active area where a coordinated effort is needed. NIGMS Council members supported the concept of a MIDAS Coordination Center. We envision the MIDAS Coordination Center to serve as a focal point for collaboration and training as well as testing and dissemination of MIDAS research products. The center will also act as the point of contact between the MIDAS network and public health organizations.
We expect to issue a funding opportunity announcement in early 2018, and we encourage the community to watch the presentation at our Council meeting to learn more about this program. We welcome your input and feedback on these plans. You can email your comments to me or post them here.
Early Notice: New NIGMS Institutional Predoctoral Training Grant Funding Opportunity Announcement
UPDATE: The new predoctoral T32 funding opportunity announcement specifically tailored for predoctoral graduate programs in the basic biomedical sciences is now available.
At the recent NIGMS Advisory Council meeting, the Division of Training, Workforce Development, and Diversity requested, and received, approval to write a new predoctoral T32 funding opportunity announcement (FOA), specifically tailored for predoctoral graduate programs in the basic sciences and designed to help catalyze the modernization of biomedical graduate education. The goal is to enable the community to develop and implement innovative approaches to education and mentoring that will more effectively and efficiently train future generations of outstanding biomedical researchers, and will allow graduate education to keep pace with the rapid evolution of the biomedical research enterprise. Taking into account the feedback we have received from various stakeholders over the past year, the new FOA will:
- Emphasize the development of a diverse pool of exceptionally well-trained scientists;
- Focus on skills development, rigor and reproducibility, inclusive and supportive training environments, and responsible conduct;
- Address conflicts in the incentive structure of the research enterprise that adversely impact biomedical graduate education;
- Encourage the use and dissemination of evidence-based, innovative educational and mentoring practices;
- Emphasize improvements in career preparation (broadly defined), and dissemination of career outcomes on publicly available sites.
The intention is not to layer additional activities onto existing structures. Instead, this funding announcement is designed to allow for a creative reinvention of biomedical graduate education that preserves the best elements, while enhancing the focus on the development of research and professional skills by trainees.
We expect to issue the new T32 FOA this fall and to receive the first applications in May 2018. The new FOA will apply to all NIGMS predoctoral T32 training grants, except for the Medical Scientist Training Program (MSTP), which will remain on the parent T32 announcement for now. We plan in the future to develop a parallel FOA that is specific for the goals of the MSTP.
We encourage the community to watch the presentation at our Council meeting and view the slide deck. As always, we welcome your input and feedback on these plans. You can post your comments below.
Why Is It important to Accurately Acknowledge NIGMS Grants in Publications?
As we’ve pointed out, it’s important to acknowledge your NIH funding in all your publications, including research articles, press releases and other documents about NIH-supported research. Your Notice of Award includes information about such acknowledgements (also see Requirements for Acknowledging NIH-Supported Research and Attribution of NIH/NIGMS Support).
If you have more than one NIGMS or NIH award, you should only cite the grant(s) that supported the research described in the publication. The specific aims should be the determining factor. This would apply even in cases where one of the authors on the article (e.g., a technician) works on multiple projects and is paid through multiple grants, or when equipment used in the reported work was purchased on a different grant.
Acknowledging multiple awards in a publication may be taken as an indicator of scientific overlap among the cited projects. This becomes important when your next application is being considered by reviewers, NIGMS Advisory Council members and NIGMS staff. For example, when considering support of research in well-funded laboratories, our Advisory Council expects the Institute to support projects only if they are highly promising and distinct from other funded work in the laboratory.
So, please take a moment to make sure that you are citing your grants accurately in your publications and avoid pitfalls when you send in your next application.
Early Notice: New Program to Support Collaborative, Team-Based Science
UPDATE: We thank the community for its initial feedback as we continue to develop plans for this program, which will support research within the NIGMS mission (including a limited number of clinical areas). The program will offer comparable levels of support as the program project (P01) mechanism, but its structure will be quite different. In addition to the capacity building and AIDS-Related Structural Biology program centers, we will continue to support the Biomedical Technology Research Resource centers (P41), Mature Synchrotron Resources (P30), and select coordinating or resource centers in areas of high strategic need.

At its January 2017 meeting, our Advisory Council endorsed a concept for a new program to support collaborative, team-based science. This initiative is the result of evaluations of our previous programs, recent research on the science of team science, and community input.
Many research questions in biomedical science can be pursued by single investigators and their close collaborators through single- or multi-principal investigator R01 grants. However, complex research questions may require the coordinated efforts of several research laboratories and closer collaborations among researchers with diverse areas of expertise. NIGMS recognizes the importance and benefits of supporting collaborative research teams when these are necessary to achieve important scientific breakthroughs or new understanding of phenomena.
NIGMS’ new Collaborative Program Grant is designed to support highly integrated, multidisciplinary research teams of three to six investigators who will address complex research questions, train and mentor new scientists, and impact scientific problems that would benefit from coordinated research support. The key application requirements are a single, integrated research program without subprojects and a multiple-principal investigator management plan. We expect to issue a funding opportunity announcement by the summer and, beginning in 2018, we will make four to six awards per year with annual direct costs ranging from $500,000 to $1.5 million. We plan to phase out our use of the P01 and most of our other center mechanisms. We will continue to support capacity building centers, such as those of the Institutional Development Award (IDeA) program, and to support the AIDS-Related Structural Biology program centers.
We encourage the community to watch the presentation at our council meeting, and we welcome your input and feedback on these plans. You can email your comments or post them here.
New NIGMS Technology Development Program Announcements
We would like to tell you about two new technology development funding opportunity announcements (FOAs) recently published in the NIH Guide. We previously wrote about the approval of these programs by our Advisory Council. They are part of an ongoing effort to facilitate early stage, investigator-initiated work to create or improve tools for biomedical research. We think the two FOAs briefly described below will stimulate early stage technology research and development by allowing scientists to focus on making the technology work before they begin to apply those tools to biomedical research questions.
Exploratory Research for Technology Development (PAR-17-046): This program will support modest 2-year R21 grants to develop a new technology or radically improve an existing one. Projects will be high-risk and have no preliminary data. The proposed technology should be justified by a significant biomedical research need, but the proposal should not include the application of the technology to a biomedical problem—it should focus on technology development.
Focused Technology Research and Development (PAR-17-045): This program will support R01 grants that are entirely focused on the development of an emerging technology with a strong potential to impact biomedical research. The program will not allow inclusion of a significant biomedical research problem because the technology will not be ready for that until the project is over. These grants will be renewable only once.
The deadline for the first round of applications is February 16, 2017.
To help investigators determine which technology development program is right for their project, we’ve posted a decision tree on the NIGMS website. It includes descriptions of the programs designed to support all stages of technology development.
We welcome questions or comments about these FOAs or our technology development programs in general.