I’d like to remind the NIGMS community of our clearinghouse site of materials designed to teach rigorous experimental design and enhance data reproducibility. There, you’ll find links to a number of online resources including:
Continue reading “NIGMS Resources on Rigor and Reproducibility”Tag: Rigor and Reproducibility
Administrative Supplements for NIGMS Predoctoral T32 Grants to Develop Curricular and Training Activities
To continue our efforts to catalyze the modernization of biomedical graduate education, we invite eligible NIGMS-funded T32 predoctoral training programs to submit administrative supplement requests (NOT-GM-19-015) to develop new curricular and training activities that enhance the program’s ability to: 1) provide graduate trainees with a strong foundation in research design and methods in areas related to conducting rigorous and transparent research to enhance reproducibility; 2) prepare students for diverse careers in the biomedical research workforce; 3) develop the knowledge and skills of trainees to enhance laboratory safety; and 4) develop the technical, operational, and professional skills of predoctoral biomedical researchers.
Administrative Supplements for NIGMS Predoctoral T32 Grants to Develop Curricular and Training Activities
To continue our efforts to catalyze the modernization of biomedical graduate education, we invite eligible NIGMS-funded T32 predoctoral training programs to submit administrative supplement requests to develop new curricular and training activities to enhance the program’s ability to: 1) provide graduate trainees with a strong foundation in research design and methods in areas related to conducting rigorous and transparent research to enhance reproducibility (PA-18-756); 2) prepare students for diverse careers in the biomedical research workforce (PA-18-757); 3) develop the knowledge and skills of trainees to enhance laboratory safety (PA-18-758); and 4) develop the technical, operational, and professional skills of predoctoral biomedical researchers (PA-18-759).
Grantees should consider the following before applying:
Funding Opportunity for Development of Training Modules to Enhance the Rigor and Reproducibility of Biomedical Research
It’s crucial that the results of NIH-supported biomedical research are reproducible, unbiased, and properly validated. Establishment and use of rigorous and reproducible approaches require appropriate and sustained training of researchers and students. In 2014, we announced a funding opportunity to develop, pilot, and disseminate training modules to enhance data reproducibility. The products of these grants are posted on the NIGMS website as they become available, together with other relevant training modules about conducting rigorous and reproducible research.
We’ve just reissued a funding opportunity announcement (FOA) to support the development of additional training modules in three areas that build upon and extend those targeted through the previous FOA. The three new areas of emphasis are: 1) How scientific culture, organization, and incentives influence the rigor and reproducibility of biomedical research; 2) Good laboratory practices and record keeping; 3) Advanced experimental design and analysis.
Your Perspectives: Catalyzing the Modernization of Biomedical Graduate Education
NIGMS actively supports efforts to catalyze the modernization of biomedical graduate education. We have undertaken a number of initiatives to stimulate this process, including hosting a symposium to showcase innovations in biomedical graduate education and providing administrative supplements to T32 predoctoral training grants to enhance rigor and reproducibility, career development and skills development.
On June 8, 2016, we took another step to encourage such change with the release of a Request for Information (RFI) seeking input on how our institutional predoctoral training grants program can be used to promote innovations in training. The RFI asked members of the community to weigh in on the strengths and weaknesses of the current system, the skills the next generation of graduate students should acquire, barriers to change and strategies to promote change through our institutional predoctoral research training grants.
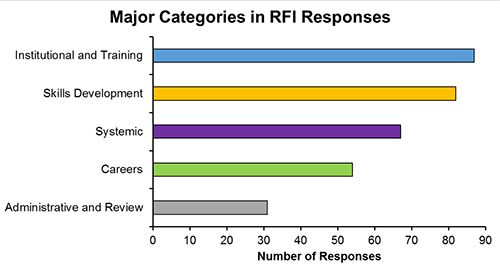
We received 90 unique responses from stakeholders ranging from students and faculty to institutions and professional societies. Themes represented in the responses were organized around five major categories:
- Institutional and training-related issues,
- Skills development,
- Systemic issues within the research enterprise,
- Careers, and
- Administrative and review issues.
While NIGMS recognizes that those who responded to the RFI are unlikely to represent a random subset of the individuals and organizations who have a stake in graduate biomedical education, these responses provide insights regarding how members of the extramural community view the current challenges and opportunities in graduate biomedical education. As such, these comments will inform NIGMS’ ongoing efforts to catalyze the modernization of graduate education through a new predoctoral T32 funding announcement, which is currently under development. For more details about the analysis, we encourage you to explore the report.
Partnering with Professional Societies
Not long ago, Jon Lorsch and I and several other NIGMS staff met with the leadership of one of the professional societies that represents many of our grantees. It was an opportunity to discuss NIGMS’ policies and grant mechanisms, hear about challenges that investigators face, and share ideas about how the biomedical research and training environment can be improved.
Meetings of this kind are not unusual, but they are just one of the ways we interact with the society partners related to NIGMS’ mission and, through them, communicate with their members. Another way is by attending the societies’ scientific meetings, where our staff learn about the latest research in the field, conduct grantsmanship workshops, and answer questions about the funding process.
The professional societies help us disseminate—and receive—information. For instance, they share our notices about funding opportunities and changes in NIH policies as well as respond to our requests for information. Leadership from the professional societies attend the open sessions of our Advisory Council meetings and sometimes speak during the public comment period, enhancing the exchange of information between the Institute and our constituency.
We also collaborate with professional societies on specific activities. Recent examples include meetings convened by FASEB on rigor and reproducibility and by ASBMB on research training. With ASCB, we co-organized the Life: Magnified exhibit, which brought biomedical science to a public place.
We greatly value our interactions with the societies and invite suggestions for additional ways we can partner.
Give Input on Strategies for Modernizing Biomedical Graduate Education
We’ve been examining how best to support the modernization of graduate education at the national level to ensure that trainees gain the skills, abilities and knowledge they need to be successful in the biomedical research workforce.
We’re involved in a variety of efforts. For example, we and other NIH institutes and centers provided support for the development of training modules on rigor and reproducibility. We encouraged graduate programs at institutions that receive predoctoral T32 support from us to make their alumni career outcomes publicly available to prospective and current students. We’ve also offered administrative supplements to predoctoral T32 training grants to support innovative approaches in the areas of rigor and reproducibility, career outcomes and graduate education. In April, we held a symposium covering these and other topics in graduate education. Finally, we plan to write a new predoctoral T32 funding announcement.
We’re now soliciting input from the biomedical research community and other interested groups in response to a new request for information (RFI) on strategies for modernizing biomedical graduate education. We’d like to know your thoughts on:
- Current strengths, weaknesses and challenges in graduate biomedical education.
- Changes that could enhance graduate education to ensure that scientists of tomorrow have the skills, abilities and knowledge they need to advance biomedical research as efficiently and effectively as possible.
- Major barriers to achieving these changes and potential strategies to overcome them.
- Key skills that graduate students should develop in order to become outstanding biomedical scientists and the best approaches for developing those skills.
- Potential approaches to modernizing graduate education through the existing NIGMS institutional predoctoral training grants.
- Anything else you feel is important for us to consider.
Responses can be submitted via an online form and can be anonymous. They can also be emailed to modernPhD@mail.nih.gov. The due date for responses is August 5, 2016.
Small Business Opportunity to Develop Cell Line Identification Tools
Misidentified and contaminated cell lines are believed to be a significant cause of irreproducible and non-generalizable research results. Although this issue has been widely discussed, including on this blog, surveys have shown that many researchers find the costs, time and effort of cell line authentication to be barriers to using it as a routine quality-control measure. There’s a new trans-NIH Small Business Innovation Research (SBIR) initiative that aims to reduce these barriers and make cell line authentication affordable and routine.
Along with other parts of NIH, we’re participating in a funding opportunity announcement (FOA) to support SBIR projects focused on developing novel, reliable, rapid and cost-effective tools to assist researchers in confirming the identity and/or sex of the cells that they use in their work. The FOA will also support the development of tools for cases in which there are currently no good methods of identification, such as distinguishing between cells derived from inbred mouse lines. We encourage applications from all eligible organizations. Standard application due dates apply.
If you’re interested in applying and would like more information, you can email me, my NIGMS colleague Zhongzhen Nie or other NIH program staff listed in the FOA. We look forward to receiving your best ideas for developing and commercializing new cell line identification tools.